

Higher and foundation tiers
Once you have found the relative formula mass of a compound it is possible to calculate the percentage by mass for each element present in the compound. Simply use the formula shown below:

Example 1: Find the percentage by mass for each element present in a molecule of carbon dioxide (CO2).
Ar of C=12 Ar of O=16
Start by calculating the Mr of carbon dioxide:
Mr of carbon dioxide = (Ar of carbon) + (2xAr of oxygen)
= 12 + 32
=44
Mr of CO2= 44.
Now using the formula above simply put in the values for the Ar and Mr:

Just check once you have done all your calculations that the total % composition for all the elements in the compounds adds up to 100%. If it does not then you have made an arithmetic error in your calculation.
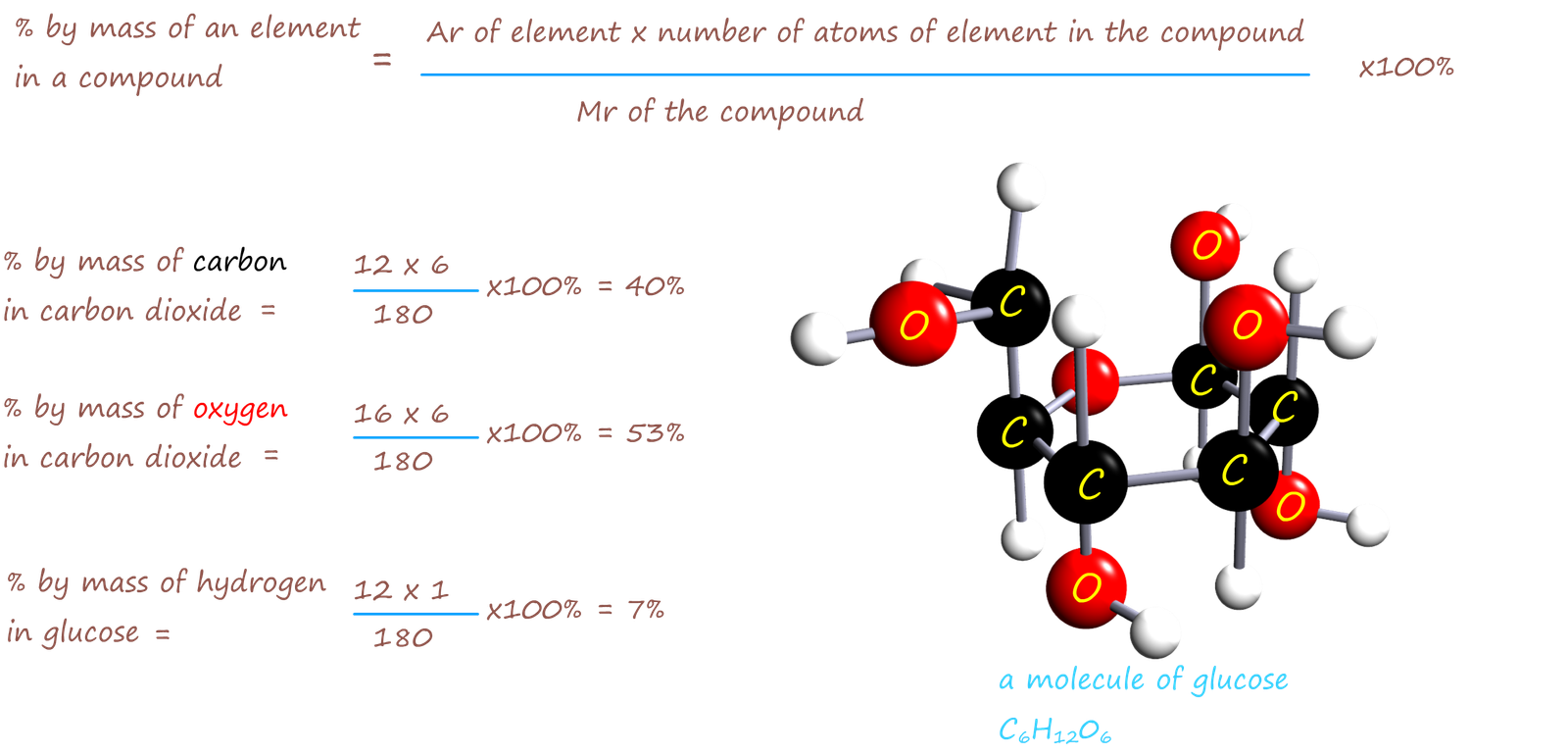
Glucose has the formula C6H12O6. Calculate the % by mass
of C, H and O in glucose.
Use the same method as above to find the % composition by mass for each element in a molecule of glucose.
Ar of C=12 Ar of O=16 Ar of H=1
Mr of glucose = (Ar of carbon x6) + (Ar of hydrogen x12) + (Ar of carbon x6)
= 72 + 12 + 96
=180
To review your understanding on how to work out the percentage by mass of an element in a compound try the activity opposite or answer the questions in the worksheets below, simply click the links to access the worksheets: